At Bryter Lab, we are always experimenting with new techniques for market research. We’re passionate about providing the most insightful answers to our clients’ questions, and often this means using advanced and innovative methods.
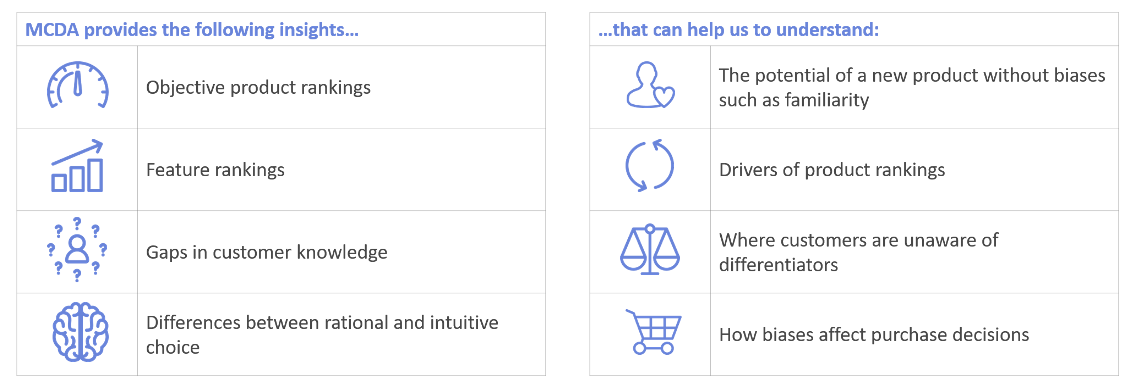
MCDA is a highly flexible and powerful analytical approach that unpicks multi-level decision-making. It works by breaking down the available options into their component parts, and then comparing these parts. This structures the decision-making process. Ideal for understanding prescription decision-making. It can also be used to understand the features of a product that are most important to customers, and the features that are driving a high product rating.
For example, when considering which treatment to prescribe, doctors consider features such as efficacy, safety, and dosing. With an MCDA, doctors rate the importance of these features, and then rate each of the treatments on each feature.
Which features are more important? How high does a treatment need to score on each feature in order to be considered? What level of safety is acceptable? MCDA can give us numerical answers to these questions, without requiring the large sample size of a conjoint.
MCDA Analysis of Biologics in Plaque Psoriasis
To demonstrate this, we asked Dermatologists and Physician Assistants (PAs) their views on four dermatology products: Cosentyx, Humira, Taltz and Stelara.
The respondents were required to indicate the relative importance of four product features: mechanism of action, efficacy, recommended ages, and frequency of administration.
MCDA revealed that the difference between the top two levels of efficacy, those of Taltz and Cosentyx, was telling for dermatologists but being lost on derm PAs. As such, Lilly the manufacturer of Taltz, perceived as the most efficacious product by dermatologists would be advised to educate PAs about the difference in efficacy between their product and Cosentyx.
The MCDA also showed that overall ratings were most influenced by efficacy, highlighting it as the most important driver.
Benefits of MCDA
In a standard rating question, doctors are inclined to rate products based on past experience. Their responses can often be biased by factors such as how comfortable they are with a product. Breaking down the rating using an MCDA allowed us to gain more objective insight into what they really thought about each product.
MCDA can be used in both quantitative and qualitative research. It is quick and works with small sample sizes, making it cost effective for research into rare or orphaned conditions.
These advantages also make MCDA an attractive method for assessing pharmaceutical products in the early stages of development. MCDA can help to drive the success of a new product by providing an understanding of which product features marketing should focus on.
In a nutshell, MCDA makes it possible to structure the process of product rating. This structured data can reveal a range of insights that a simple rating cannot. Here at Bryter we are now using MCDA in relation to both pipeline and launched products as one of a number of innovative methods in our toolkit. Don’t hesitate to drop us a line about how this could help you on your next project.


.jpg)


